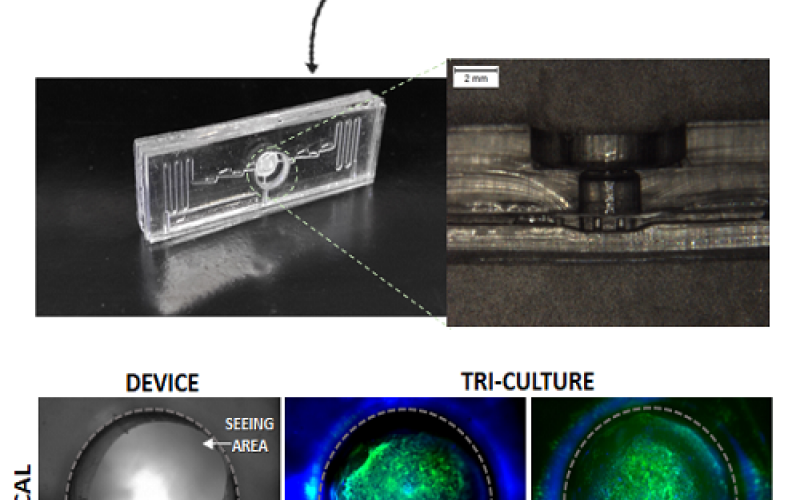
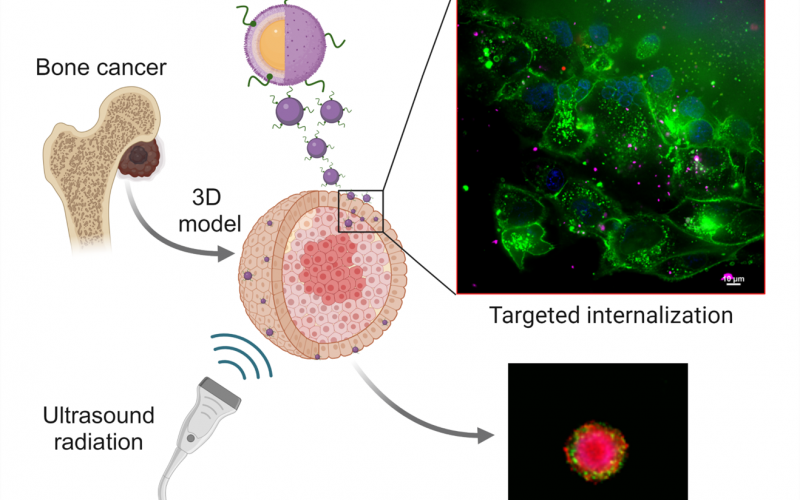
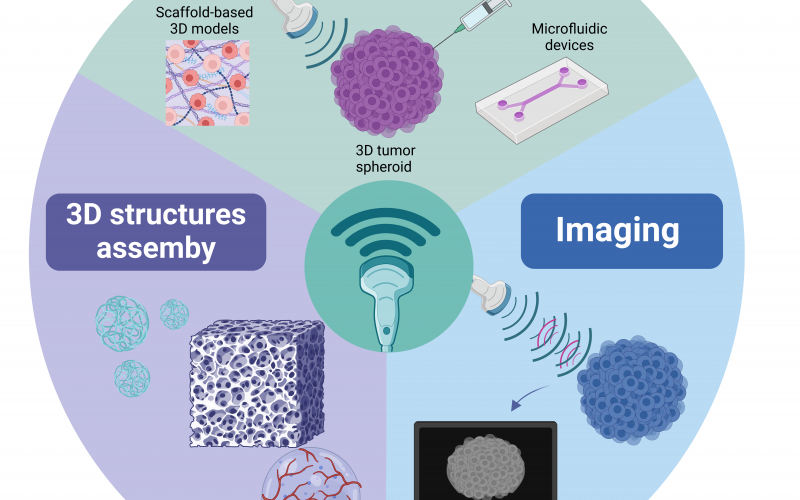
- Centro Interuniversitario per la Promozione dei Principi delle 3R nella Didattica e nella Ricerca
3Rs lab training - Alterox
La Alterox academy organizza diversi eventi nell'ambito delle 3R.
Di seguito la lista dei prossimi 3Rs lab training. Per ulteriori informazioni, si visiti il sito http://academy.altertox.be/
Title: Informing Policy Making Through Evidence
Date: 11 July 2019
Host: Altertox
City: Brussels
Country: Belgium
Registration link: https://skills4science.eventbrite.com
Title: Becoming Sketchnote Ninja
Date: 12 July 2019
Host: Altertox
City: Brussels
Country: Belgium
Registration link: https://skills4science.eventbrite.com
Title: PBPK Modeling and quantitative in vitro-in vivo extrapolations
Date: 3-4 October 2019
Host: RIKILT Wageningen University and Research
City: Wageningen
Country: Netherlands
Registration link: https://wur-training-altertox2019.eventbrite.com
Title: Novel In Silico Models for Assessment of Cosmetics - Practical Applications
Date: 17-18 October 2019
Host: Mario Negri Institute
City: Milano
Country: Italy
Registration link: https://marionegri-training-altertox2019.eventbrite.com
Title: Good In Vitro Methods In Practice (GIVIMP)
Date: 6-7 November 2019
Host: RISE - Research Institutes of Sweden
City: Gothenburg
Country: Sweden
Registration link: https://givimp-training-altertox2019.eventbrite.com
Title: In Vitro Lungs Model
Date: 14-15 November 2019
Host: Epithelix
City: Geneva
Country: Switzerland
Registration link: https://epithelix-training-altertox2019.eventbrite.com
Title: Acute and chronic cardiotoxicity: CiPA (in silico & in vitro) assays
Date: 14-15 November 2019
Host: Ncardia
City: Leiden
Country: Netherlands
Registration link: https://ncardia-training-altertox2019.eventbrite.com
Title: Skin Sensitization
Date: 21-22 November 2019
Host: University of Trier
City: Trier
Country: Germany
Registration link: https://trier-training-altertox2019.eventbrite.com
| Allegato | Dimensione |
|---|---|
| 31.35 KB |